|
|
Issue
40: November 2007
Check out the following in our on-line Virtual Shop :
Table of Contents
Nestlé boycott news
Editorial: New-look newsletter and gains in the PhilippinesOur printed newsletter is sent to members (you can opt to receive it electronically only if you prefer). It can also be viewed here and downloaded as a pdf file. You may notice that the printed version of the newsletter has shrunk in page size, but grown in number of pages. It is now in full colour and printed on recycled paper. We hope you like the new format. It has been forced upon us by changes to postal rates in the UK, which make the old size newsletter far more expensive to send out. We will be developing the style to fit the new page size and the possibilities of colour and welcome your suggestions. In this issue we report on the important protection won in the Philippines, where the Supreme Court has lifted a restraining order on Department of Health regulations for the marketing of baby foods. It took an international campaign to expose industry pressure on the President. We also focus on the UK law campaign where we are urging policy makers to follow the Philippines lead and put health before trade. Plus the usual nuggets of news from around the world and updates on the Nestlé boycott. UK law campaignConsultation on strengthening the UK law
Baby Milk Action convened the Baby Feeding Law Group (BFLG) in 1997 to bring health professional and lay organisations together to campaign for the implementation of the International Code of Marketing of Breastmilk Substitutes and subsequent, relevant World Health Assembly Resolutions in UK law. In 2004, the BFLG won a commitment from the Government in its White Paper Choosing Health to strengthen the UK Law and the European Union Directive on Infant Formulae and Follow-on Formulae from which it derives. During the negotiations in Brussels to revise the EU Directive, the UK did push for most of the BFLG demands. However, the Commission, which chaired closed unminuted Expert Meetings, refused to accept the UK’s evidence that follow-on milk and other promotion is undermining breastfeeding. The revised Directive (2006/141/EC) adopted in July 2006 is full of loopholes but must be implemented by 2008. This issue highlights the conflict between trade and health at the heart of EU policy making. One aim of the EU is to harmonise trade rules and encourage the free movement of goods. But the EU Treaty also states that: “A high level of human health protection shall be ensured in the definition and implementation of all Community policies and activities.” Baby Milk Action and UNICEF UK met the EU Commission’s Head of Legal Affairs and Commission staff to discuss the Directive and the BFLG will meet the Public Health Minister, Dawn Primarolo in November. Baby Milk Action has submitted a complaint about the Commission to the European Ombudsman (See below).
BFLG members
Save the Children, UNICEF UK and NCT also sent a joint submission, A weak Formula for legislation, and hosted a web-based cartoon, Little Jack, about claims. Click here. Health experts demand stronger regulationsGovernment expert advisors call for stronger regulationsThe UK Food Standards Agency (FSA), an independent government agency which reports to the Health Minister, began a 3-month consultation on its draft proposals for the new formula regulations in July (Click for England, for Wales, for Scotland, for Northern Ireland). The FSA has told the BFLG in meetings that it will implement the Code if there is a legal way to do do more than simply transposing the text of the EU Directive. The BFLG report argues that Member States can do this if it is in the interests of health. Paragraph 27 and Art 1 of the Directive not only permit Code implementation but could be said to require it. The FSA received 1,341 responses, including the BFLG 40-page submission setting out the legal case for strengthening the legislation and the economic and social benefits that could be expected. The UN Rapporteur for the Right to Food (Jean Zeigler) and the Government’s Scientific Advisory Committee on Nutrition (SACN) also wrote as follows:
See:
Why the law needs to changeBFLG monitoring project presents evidence of malpracticeArticle 11.3 of the International Code requires that companies must “ensure that their conduct at every level conforms” to the Code’s provisions independently of government measures to implement them. This means companies should not be promoting their products or making direct or indirect contact with parents and should limit their activities to producing safe, clearly labelled products and scientific, factual information for health workers. The BFLG monitoring project shows that companies violate the Code and Resolutions in a systematic way and that the weak UK law is too full of loopholes to stop this.
Claims such as ‘contains prebiotics’ could eventually be permitted by the Nutrition and Health Claims Regulations (1924/2006) on follow-on milks and baby foods - based only on evidence relating to adults.
Companies argue that claims such as “For Hungry babies” are not nutrition claims. The BFLG report has more examples. “Little Jack” the online cartoon by UNICEF, Save the Children and the National Childbirth Trust shows how claims mislead. Voiceover by Mariella Frostrup. View here.
Safer formula campaignCompanies do not inform parents of risksNestlé cleared in Belgium case - but cases settled in the USThe risk of contamination of powdered infant formula with Enterobacter Sakazakii came to our attention in 2002 when a 5-day-old child, Natan Geerinck, died from meningitis linked to Nestlé’s Beba formula in Belgium (see Update 31). In October 2007 a Belgian judge rejected a legal action brought by Natan’s parents against staff at the hospital and Nestlé. Nestlé had not provided warnings on labels about the known risks of contamination and the need to prepare the product with water that is greater than 70 deg. C, but it had complied with legislation.
EU ‘hopes for the best’ as it blocks global safety standards
The EU representative, Basil Mathioudakis, said: “Let’s adopt the Standard as it is and hope for the best.” In Delhi in November the EU also tried to stop a requirement that follow-on formulas should meet the same microbiological criteria as infant formulas. An expert WHO/FAO consultation on follow-on formula use will now follow. Download the guidelines at: Philippines requires warningsBelow we report on the decision by the Supreme Court in the Philippines. Amongst other things, the judge ruled in favour of the Department of Health’s proposed warnings on the labels of powdered formula. The industry had attempted to strike down the requirement that it inform formula users of risks. The ruling said:
...and so will South AfricaThe South African Government’s draft proposals for its new law contain many excellent provisions including good warnings about contamination. To comment see ‘Codewatch’ on our website. ...but the UK soft pedalsThe UK FSA guidance on the preparation of powdered formula, issued in 2005, was followed in January 2007 with research on parents’ understanding of the risks. The FSA has now proposed a voluntary agreement with companies to warn parents that the products are not sterile. Based on its monitoring, the BFLG considers that a strong statutory requirement will be essential. In 2007 UK companies issued new labels. Only Hipp warns that the product is not sterile but instead of recommending mixing with water above 70 deg. C as set out in the guidelines, it recommends 50-60 deg. C. See: Baby Milk Action press release 10 August 2007: New UK formula labels lack correct information - calls for better warnings and instructions. FSA guideance to parents: FSA research on parents' understanding: You can’t trust company carelinesSpot monitoring conducted by Baby Milk Action shows company carelines are being used to circumvent the ban on advertising of infant formula in the current UK law and provide misleading and dangerous information. Carelines are promoted heavily in advertisements, fliers, mailings to parents and on the internet. Parents are directed to them for information on infant care. Baby Milk Action called the carelines in the role of a confused parent wanting to know the difference between the formulas on the market and the particular health claims made about the company’s product. As a subsequent question, we commented on the notice on Hipp labels that powdered infant formula is not sterile and asked if the same was true of the company’s products and if any action was needed to reduce risks. Aptamil: room temperature
Asked about sterility and the need to use hot water, she said it wasn’t necessary:
This is incorrect. Thirty minutes is not the optimum time, it is on the limit of that recommended. The FSA guidance for parents states:
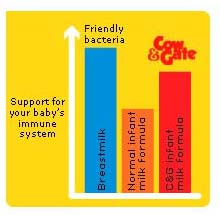
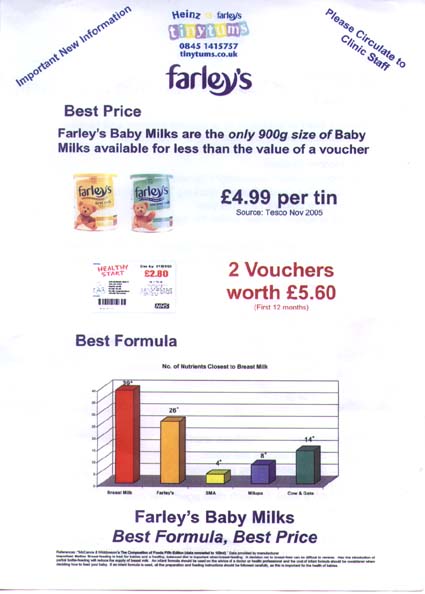
Cow & Gate: cold waterThe Cow & Gate advisor was asked about the claim that its formulas contain prebiotics and said that prebiotics are present in breastmilk, support the natural immune system and provide food for friendly bacteria. Asked if they are the same as in breastmilk “Yes, prebiotics are in breastmilk” and added that only Cow & Gate and Aptamil have them. Asked whether Cow & Gate formula was sterile, she said “No formula is sterile.” Asked if that was a problem, she said: “Makes no difference.” She advised: “Prepare bottles of sterile water in advance. That stays fresh without refrigeration for 24 hours. Take from the bottle and mix. That formula is good for an hour.” Farley’s: no temperature adviceAsked about the claim on new Farley’s formula labels saying it contains ‘Omega-3 LCPs’ (see above) Farley’s advisor said: “It helps develop their brain in their first few months” claiming: “The Government stresses the importance of Omega 3.” She said only Farley’s had LCPs. Asked if this made it closer to breastmilk than competitors, she said: “Farley’s is the closest. Yes it is.” Asked about the need to use hot water as powdered formula is not sterile, her advice was: “Heat the water and let it cool. I don’t know if it gives the temperature. It says 30 minutes.” The FSA guidance says no more than 30 minutes. Asked if using hot water was important, she said: “It mixes better” and had nothing to do with sterility. Hipp dismisses FSA guidanceThe Hipp Careline advisor did not have an answer to questions on sterility, so the Nutritionist phoned back. She said Hipp did not follow the FSA guidance for parents of using water above 70 deg. C because “you kill the protein and it would be dangerous to hold it.” When asked about the FSA guidance, she said water at 70 deg. C would cool when it was mixed with the powder so “the temperature would be lowered anyway.” She claimed “no-one does it at 70 deg. C” and “we certainly have no problems with bacteria.” SMA: not sterile after openingThe SMA advisor was asked about its ‘new protein balance’, promoted on labels. She said: “It makes it closer to the protein found in breastmilk.” She was asked how SMA could be the closest to breastmilk when the Aptamil label and advertising says it is the closest and replied: “Ours is balanced. It is closest.” Asked about sterility, she said: “No formula is sterile if it is exposed to the air.” Despite Hipp claiming no company says to use water at 70 deg. C, SMA did just that: “We tend to say 70 Degrees. These are new Department of Health Guidelines. It can destroy any bacteria that may be in the powder. Can no longer use water cooled to room temperature. We say boiled for 30 minutes in the kettle.” UNICEF and WHO adviceWHO recommends mixing formula with water at 70 degrees C. WHO experts say this is the single most effective decontamination step which could reduce the risk 10,000-fold. UNICEF UK Baby Friendly multi-lingual leaflets are also available. See: WHO Guidance can be downloaded from: http://www.who.int/foodsafety/publications/micro/pif2007/en/index.html Which is the best formula?It is not only information on Carelines which is confusing or simply wrong.
Watching EUEuropean formulas: a mass uncontrolled trial?Although the revised EU Directive (above) in many ways improves the essential composition of formulas - a reason why so many Member States were keen to adopt it - it also allows companies to add “other food ingredients, as the case may be.” There is no requirement that the ingredients are evaluated by an independent scientific body prior to introduction onto the market - even though the majority of EU member States and the EU’s advisory body, the Scientific Committee for Food, called for this safeguard. If manufacturers introduce a new infant formula they only have to submit a label to the authorities - and that is all. There is no notification procedure at all for follow-on milks. To make matters worse, follow-milks may be able to carry claims which are supported only by research on adults. Breastmilk substitutes can be the sole source of nutrition during a critical period of rapid growth and development. Minor modifications can have major effects on infant health. The Report of the Scientific Committee on Food, 2003, identifies problems that have occurred with the introduction of modified infant formulae. Examples include reduced protein availability with impairment of growth; trace element deficiency with severe clinical disease; chloride deficiency with long-term neurological damage and thiamine deficiency with severe clinical disease, including neurological damage and several cases of infant death. The fact that the EU Directive failure to include a rigorous pre-market authorisation plays into the hands of the companies who are prepared to add any ingredient - before its safety has been properly evaluated - simply to gain competitive advantage. This is equivalent to a mass uncontrolled trial. (See Protecting breastfeeding - Protecting babies fed on formula p11).
EU Commission - enough is enoughBaby Milk Action has submitted a complaint to the European Ombudsman about the Commission’s handling of the Directive 2006/141/EC specifically by Basil Mathioudakis, the Head of the Food Law, Nutrition and Labelling Unit in the Health and Consumer Protection Directorate (DG SANCO). Update readers will know our concerns about Mr Mathioudakis, who joined the Commission in 1982 soon after MEPs and Member States first called for the implementation of the International Code in Europe. We complain that Mr Mathioudakis has, among other things: misrepresented our meeting with him, failed to Chair the Expert meetings in an objective way; failed to take on board the serious concerns of Member States about the need to protect public health and implement the Code and misrepresented the proceedings of the expert meetings.
Parliamentarians ask WHOMEPs Catherine Stihler, Richard Howitt and Glenys Kinnock, Lord Avebury and many MPs have been helping with our campaign. In answer to a request from Catherine, WHO explained the importance of the International Code in the European context, confirming it is:
Research newsGenetic link found to IQFrom a gene study of children born in 1972-73 in New Zealand and 1994-95 in England, British researchers have found that mothers’ milk in the first few months of life can boost children’s IQ by seven points.1 A genetic link in 9 out of 10 breastfed children is thought to enable them to benefit from the Long Chain Polyunsaturated Fatty Acids (LCPUFAs) in breastmilk. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism Journal Proceedings of the National Academy of Sciences. http://www.pnas.org/cgi/content/abstract/104/47/18860 The jury is still out on LCPUFA supplementation of formula. The 2001 Cochrane Library review of studies on adding LCPUFAs to formula concluded that there was little evidence to support a benefit for visual or general development of term infants. The 2004 UK SACN Advice on Fish Consumption Benefits and Risks found a benefit for visual development in pre-term infants but less evidence of effects on term infants. The largest trial failed to demonstrate an effect (also see above). The BFLG position is that if an ingredient has been unequivocally demonstrated to be essential and beneficial by an independent review of data, including a substantial proportion of independently-funded research, it should be a mandatory ingredient in all formulas, not flagged up with a claim for commercial advantage. Breastfeeding and CancerThe World Cancer Research Fund (WCRF) report Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective published in November states that there is strong evidence that breastfeeding protects mothers against breast cancer and babies from excess weight gain which is linked to increased risk of cancer. The report of a panel of 21 world-renowned scientists is the most comprehensive report on cancer prevention ever produced and the first to give a specific recommendation to breastfeed to reduce risk of cancer :
http://www.wcrf-uk.org/research_science/expert_report.lasso Infant feeding and ObesityThe Government Office for Science Foresight programme report Tackling Obesities: Future Choices, published in October, cites the role of breastfeeding in tackling the obesity epidemic: “There is evidence that the period soon after birth is a time of metabolic plasticity. Factors in the environment, such as nutrition, can have long-lasting consequences in that they appear to set the baby on a particular developmental trajectory.” Despite uncertainties surrounding the evidence and the need for additional research, weight gain in early life appears to be critical and the fact that breast-fed babies show slower growth rates than formula-fed babies may contribute to the reduced risk of obesity later. http://www.foresight.gov.uk/Obesity/Obesity_final/Index.html
Order this poster in our on-line Virtual Shop. Update 40 - continued | ||||||||||||||||||||||||||||||||